Female Hemophilia In A Set Of Triplets
Introduction: Hemophilia A (HA) and B (HB) are the most common inherited bleeding disorders. Gene sequencing identifies specific mutations and their associations with plasma factor levels and clinical phenotypes. Both HA and HB are X-linked recessive disorders, where the female carriers pass the gene onto their descendants. Males who inherit the mutated gene from their mothers develop the disease, whereas females are carriers due to having one normal gene, they typically do not display the characteristic features of hemophilia and have moderately low factor VIII levels of less than 40%. Female carriers may have bleeding symptoms, yet they do not experience as severe morbidities as the male hemophilia patients, due to having higher plasma factor VIII levels. Rarely female carriers can be severely affected and may have plasma factor VIII activities comparable to those with severe hemophilia. Here we are presenting a set of triplets, where the male infant and one of his sisters have severe hemophilia while the other sister is a hemophilia carrier.
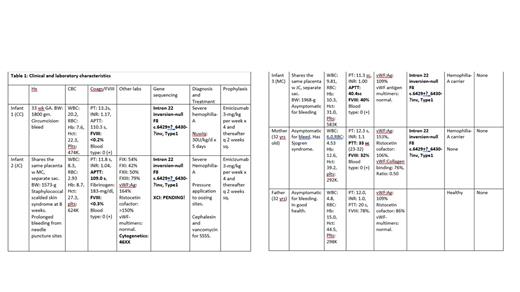
Methods: A set of triplets a male and two female neonates were born after 33 weeks of gestation to a healthy mother. Female neonates were dichorionic but shared the same placenta. The male infant had a circumcision at six weeks of age, which was complicated with prolonged bleeding. He was brought to the hospital and was diagnosed with severe HA. (Table.1).
Infant #2 was admitted to the hospital for skin rash at age 8 weeks. She had staphylococcal scalded skin syndrome and was treated with vancomycin and cephalexin. She was noted to have prolonged bleeding from needle puncture sites. She was diagnosed with severe HA. The bleeding was controlled with tight packing. After a week of treatment her rash cleared, and she was discharged home. (Table 1).
Infant #3 She was a healthy baby with no bleeding problems. (Table 1).
Results and conclusions: We presented three infant-triplets-with factor VIII deficiency; infant #1 and infant #2 have severe HA. Infant #3 is an asymptomatic carrier. They all have the same mutation as their asymptomatic carrier mother. Infant #1 the male infant developed severe HA due to having the hemophilia allele on his “X” chromosome. Infant #3 a female infant is an asymptomatic carrier. She inherited the hemophilia allele on the X chromosome from her carrier mother and has an X chromosome bearing the normal allele, thus is able to produce factor VIII of 40%.
Infant #2 the other female infant had severe HA, with a factor VIII activity of <0.3%.
Female hemophilia is rare occurrence. Hemophilia treatment center (HTT) population profile (PP) reported that of the 1,676 females attending to HTC, 47.6% of them had less than 40% factor level, 0.48% of them had severe, 1.4% had moderate and 17.9% had mild hemophilia[Haemophilia. 2021:27(6):1037-1044. Doi:10.1111}. This can occur, in the setting of monosomy X (Turner syndrome-XO), due to having one X chromosome carrying the hemophilic gene or in the setting of compound heterozygosity: the female product of a father with hemophilia and the mother being a carrier. Similarly, hemizygosity of the hemophilic gene on both parents would result in hemophilia in their daughters.
Another mechanism that plays a role in the pathogenesis of female hemophilia is the “X chromosome inactivation (XCI). This has been described as the most common cause of female hemophilia. One of the two X chromosomes in females is randomly and permanently inactivated in cells to ensure the balance with one X chromosome of males and limit the overproduction of potentially toxic gene proteins. The degree of inactivation determines the plasma levels of the gene products, in hemophilia the factor levels.
The chromosome analysis of infant #2 revealed normal 46XX. Structural chromosomal abnormality is ruled out as she did not have Turner syndrome “XO”, or “45XY” with androgen insensitivity syndrome, nor did she have compound heterozygosity or hemizygosity of the X chromosome. The father had normal coagulation studies and factor VIII activity. Therefore, we believe infant #2 had extremely skewed XCI and the inactivation is skewed in favor of hemophilic X chromosome. This mechanism resulted in severe HA in this infant.
The infants are now seven months old and have no bleeding problems, as infant #1 and #2 are on prophylaxis with emicizumab (Hemlibra), they both have 1% factor activity by chromogenic assay and do not have inhibitors. (Table 1)
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Emicizumab (Hemlibra) and Nuwiq. Both agents were used to control the hemorrhage (Nuwiq) and prophylaxis of the infants with hemophilia A.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal